 |
Marsico
Hall Microscopes Facility
(MHMF.ORG) |
 |
|
Leica Stellaris5 Laser Scanning Confocal Microscope with WLL & UV lasers
|
|
Location: 7228A Marsico Hall
|
|
Marsico Lung Institute
|

0. Notices
- This machine is owned by the Marsico Lung Institute/Cystic Fibrosis
Research Center:
- Use is limited to approved and trained users
- Please arrange orientation, training and sign on access with Michael
- All use must be logged recording 1. User; 2. Lab(s); 3. Project(s)
including chart string field
- Machine must be started correctly with particular attention to
objective selection, z-position, stage clearance, upper limit setting
- While searching and viewing samples by eye very careful attention must be made to not focus up into the sample
holder, especially when near edges of samples
- Don't focus into slide. The stage can be buckled and the objective
damaged.
-
Booking calendar or
from
http://microscopy.unc.edu/booking
-
Machine must be shut down correctly with attention to:
- Laser shut down in LAS X software
- Careful objective cleaning if glycerol or oil used, lowering objective turret,
putting 5x or 10x objective in light path,
- Exiting LAS X software and letting
it shut down completely before logging off,
- Keying off laser emission,
turning off laser power and master power (Only after lasers have been shut
down in the LAS X software)
-
Environmental chamber supports temperature and humidity
control, CO2 (0 - 10%) - Anoxia is possible by arrangement (Nitrogen tank
will need to be ordered).
-
If environmental chamber is going to be used contact Michael about
changing stage top box, objective clearance & condenser height setting in order to prevent stage
crashing into condenser
-
Connecting to file shares (SOM server), e.g. CF Groups/shared, MHI
groups, Peds groups
1. Operating the system
- Note: Before viewing your sample the system should be powered up and
the LAS X software initialized as described below
- Quick Guide:
- Powering up:
-
 Check that microscope table is correctly floating - ~5 mm from top of aluminum to underside of rubber disk
(adjust red knobs if necessary)
Check that microscope table is correctly floating - ~5 mm from top of aluminum to underside of rubber disk
(adjust red knobs if necessary)
- Turn on power strip switches in order #1 (wait ~5 seconds) then
#2 to #4
- At master box
- 1. Turn on master switch (#5) - wait >=5 seconds
- 2. Turn on laser switch (#6)
- 3. Turn on Emission key (#7)
- Log into computer - use your local account
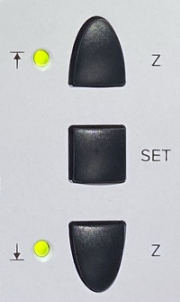
- Objective must be lowered - push and hold Z
down button (see picture on right), or set focus to course and turn
the course focus knob in the down direction.
- Place 5x, 10x or no objective in light path in order to avoid
the stage crashing an objective (turning by hand is OK)
- Ensure nothing will get in the way of stage movement - e.g. chambers, dishes,
wires, etc.
- If a stage top box is present ensure condenser will not hit
the stage top box when initializing the x-y motorized stage -
- i.e. swing the condenser back
- Once the microscope stand display has finished initializing start
the LAS X software
-
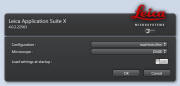 Choose
Choose
- Initialize stage if going to use programmed
x-y position control or tiling
- Find sample by eye
- Once LAS X software is running sample can be viewed
by eye
- On first startup cycle - power off the LED 3 light source off
for ~3 seconds then back on. Switch is on the back of the unit (not the
one on the front). Only need to do this once on first view.
(Its some sort of mysterious safety
procedure)
- Find and focus on sample. And set upper limit.
- Simple Scanning:
-
Select settings from choice of:
- - Load a previously saved .seq file
- - Load previously scanned
image and "Apply Settings"
- Note manually set Format, and zoom desired (Right click
and choose "Properties" on previously saved scans if you
want see these settings for consistency)
- - Dye manager (more challenging, but )
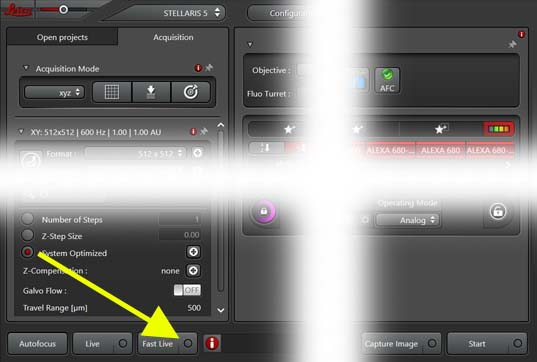
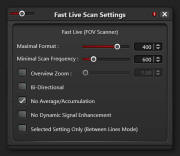
- On first scan only setup "Fast Live" options:
- Click on
circle at right of "Fast Live" button
- ON "Fast Live Scan Settings" choose "Maximum Format" of 400
and unselect "Selected Setting Only (Between Lines Mode)"
- Be careful of bleed through if doing simultaneous scanning.
(i.e. When not acquiring all channels sequentially)
- Sequential scanning is preferred to
ensure no bleed through
- Set Zoom and Pixel size to adequately sample
data
- Set averaging to reduce noise
- "Live" to view all channels
- "Fast Live" to show only selected channels
- Adjust PMT gain mostly or laser power. When viewing multiple
channels ensure multiple channels are displayed (Click on
image panel to switch between single panel & multiple panel
display)
- Save experiments as .LIF
- If Exports do not work correctly try using LAS X core free viewer
for Windows instead
- Shutting Down:
- Lower stage with course focus button on
right side of scope body
- Clean oil or glycerol off objective(s) if used. Lens tissue -
gently blot on glass lens with different areas of dry lens tissue.
Can gently rub metal body of objective.
- Power off lasers in software
- Exit LAS X software
- Put 5x, 10x or empty objective into light path
- Transfer data to a server - e.g.
SOM shared server or
mhmicroscopy share
- Wait for LAS X software to close completely <-- THIS IS IMPORTANT
-
 Turn off hardware:
Turn off hardware:
- Emission key #7
- Laser power switch #6
- Master unit switch #5
- New procedure:
Power strip off switches in reverse order #4,
#3, #2 but leave on #1 for 1 minute to allow
laser unit to cool
- then turn it off
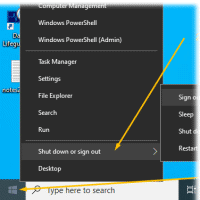
- Sign out from Windows <-- This is
important too. (Please do not just "Lock the screen"
when done)
-
Right mouse click:
Window (bottom left)
- Click "Shut down or Sign out"
- Report any problems encountered - e.g. email Michael
- Sign log book
2. The System - see here for details
http://microscopy.unc.edu/confocal/
- Inverted microscope stand DMI-8
- Laser Spot Scanning Confocal Microscope
- Lasers: 405 nm continuous wave diode & Super continuum pulsed white light laser 485 nm
(blue) to 685 nm (far red)
- Five Hybrid Photo Multipliers Tube (PMT) light detectors with emission spectral
discrimination
- High precision galvanometer z-axis positioning and motorized x-y
positioning stage
- Environmental chamber for temperature, humidity and CO2 control with
hypoxia
2.1 Lasers (excitation wavelengths):
- "UV" Diode laser 405 nm e.g. DAPI,
photo activation
- "White Light Laser"
Pulsed White Light
Laser from 485 nm to 685 nm, up to 8 wavelengths can be selected
e.g. Texas
Red, Alexa 568, Alexa 594, mCherry, will also excite Rhodamine, TRITC, DsRed & Alexa 543
2.2 Objectives:
| Objectives * |
|
| Mag. |
NA |
type |
WD |
corrections |
cover slip |
Immersion |
part no. |
DIC |
Resolution-xy+ |
resolution-z |
| Objective Wollaston |
Condenser Wollaston |
| 5x |
0.15 |
HCX PL Fluotar |
13.7 mm |
- |
|
air (dry) |
15506224 |
- |
- |
1.3 um |
? |
| 10x |
0.40 |
HC PL Apo |
2.7/2.2 mm |
- |
#1.5 |
air (dry) |
15506407 or
506285 |
- |
- |
0.49 um |
2.6 um |
| 20x |
0.75 |
HC Plan Apo |
620 um |
- |
#1.5 |
air (dry) |
15506517 |
- |
- |
0.26 um |
0.65 um |
| 40x |
1.25 |
Plan Apo |
350 um |
corr |
0.14 -0.18 mm |
glycerol |
15506422 |
E |
K7 |
0.17 um |
0.31 um |
| 63x |
1.4 |
HC Plan Apo |
140 um |
- |
#1.5 |
oil |
15506350 |
E |
K10 |
0.14 um |
0.27 um |
| L63x |
0.7 |
Plan Fluotar |
2.6 to 1.8 mm |
corr |
0.1 to 1.3 mm |
air (dry) |
11506217 |
- |
- |
0.23 |
0.72 |
** Dipping
lens, not kept on turret + Approx. resolution
with pinhole=1.0 Airy unit
WD = working distance DIC = differential
interference contrast or Nomarski
2.3 Detection:
- Emission spectral separation through a prism
- Five tunable detection bands with adjustable spectral windows before the
five Photo Multiplier Tubes (PMT) type HyD S (Hybrid GaAsP) 410 nm to 850 nm
- Standard XY scanning with zoom and rotation
- XZ rapid scanning using galvanometer stage (range +/-250 um)
- XZ scanning using microscope stand's fine focus (range mm's)
- Transmitted light and DIC (non confocal)
2.4 Direct Eye Viewing Fluorescent filters (Widefield, i.e. not confocal)
|
Epifluorescense cubes used for viewing by eye
(not confocal scanning)
|
|
|
LCD display |
Carousel Position # |
Leica cube ID |
Leica part |
Excitation Color of light |
Emission Color |
Beam splitter |
Typical fluorophores |
|
|
Analyzer |
|
(Empty) |
|
- |
- |
|
- |
|
|
LED-405 |
|
|
15525338 |
UV 405/60 |
470/40 |
455 |
DAPI, Hoechst |
|
|
GFP |
|
|
15525314 |
Blue BP470/40 |
525/50 |
495 nm |
FITC, eGFP, eYFP, FM 1-43,
CY2, Alexa 488 |
|
|
RHOD LP |
|
|
15525303 |
Green BP539/45 |
|
|
Texas Red, Rhodamine,
CY3, Alexa
555/568/594 |
|
|
(SCAN) |
|
(Empty) |
- |
- |
- |
|
|
|
| |
|
|
|
|
|
Leica fluorescent cube information |
Cube
information 3rd party
3. First time use (new user)
4. Known Bugs
- For multichannel scans need to have multiple panels open for smart gain
& smart laser adjustments correctly. (With single panel mode a hidden
channel will be adjusted). On single mode double click on single image
to restore multiple panels.
5. Viewing Files
- Leica LAS X Office - This program runs under Windows 10 is available on the
\\minsky.med.unc.edu server
- Leica LAS X core - This program runs under Windows 10 & 11 is available on the
\\minsky.med.unc.edu server
- FIJI
- Imaris
8. Leica confocal Image analysis and processing
LAS X core - Good for viewing.
Limited processing.
LAS X Office (Windows 10 only) - Good for viewing.
Limited processing.
Fiji
Volocity
7. Links
8. Reporting Problems
Please contact Michael
Leica Confocal Service and Support
866-830-0735 8:00 A.M. to 5:00 P.M.
confocal@leica-microsystems.com
serial number 820000189
Note: if you contact Leica
directly please let Michael know
Installation date: 2020-10-05
10. Dealing with software crashes and Microscope
Stand Lock Ups
- Dye manager tends to put detection wavelength too close to excitation
line. Hence reflections are prone to occur especially from grass
surfaces.
11. Applications:
Leica LAS X core 3.9 - free off line viewing and exporting
http://fiji.sc
 Check that microscope table is correctly floating - ~5 mm from top of aluminum to underside of rubber disk
(adjust red knobs if necessary)
Check that microscope table is correctly floating - ~5 mm from top of aluminum to underside of rubber disk
(adjust red knobs if necessary) Choose
Choose
 Turn off hardware:
Turn off hardware: